RESEARCH PROJECTS
Select Projects and Publications
Main protease of SARS-CoV-2 (Mpro)
The main protease of SARS-CoV-2 (Mpro) plays an essential role in the replication of SARS-CoV-2 (etiological agent of COVID-19) and thus continues to be an important protein target for therapeutic design. This has been approached from many different levels, but primarily from an in silico approach. Our lab is collaborating with a variety of academic and industrial partners to characterize and validate putative inhibitors using enzyme kinetics and X-ray crystallography. These inhibitors will then be improved upon using standard structure-based drug design methods.
Additionally, because most Mpro research solely focuses on inhibitor development, there has been a lack of basic research on Mpro structure and function, particularly in the context of the enzyme’s biological environment. Recent structural work has highlighted Mpro’s vulnerability to oxidative environments, which can arise from cellular respiration and host immune responses. These oxidative events induce large-scale structural rearrangements which impact Mpro’s structure and activity. We are investigating the consequences of these events and how this enzyme has evolved to protect itself from over-oxidation and subsequent inactivation.
Tran N, Sathish D, Barwell SAE, McLeod MJ, Kalyaanamoorthy S, Holyoak T, and Ganesan A. “The H163A mutation unravels an oxidized conformation of the SARS-CoV-2 main protease”. Nat. Commun. (2023). doi: 10.1038/s41467-023-40023-4.

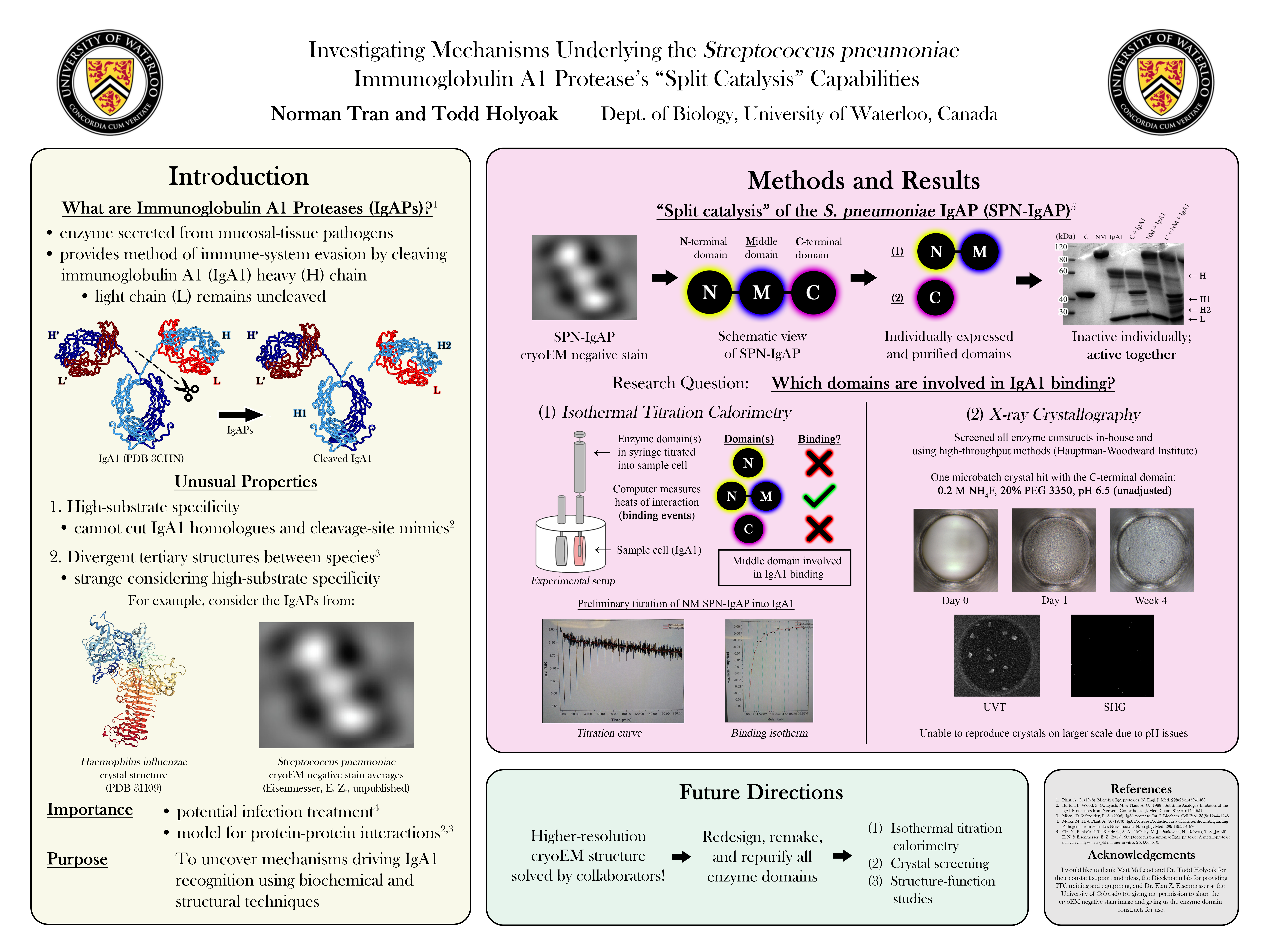
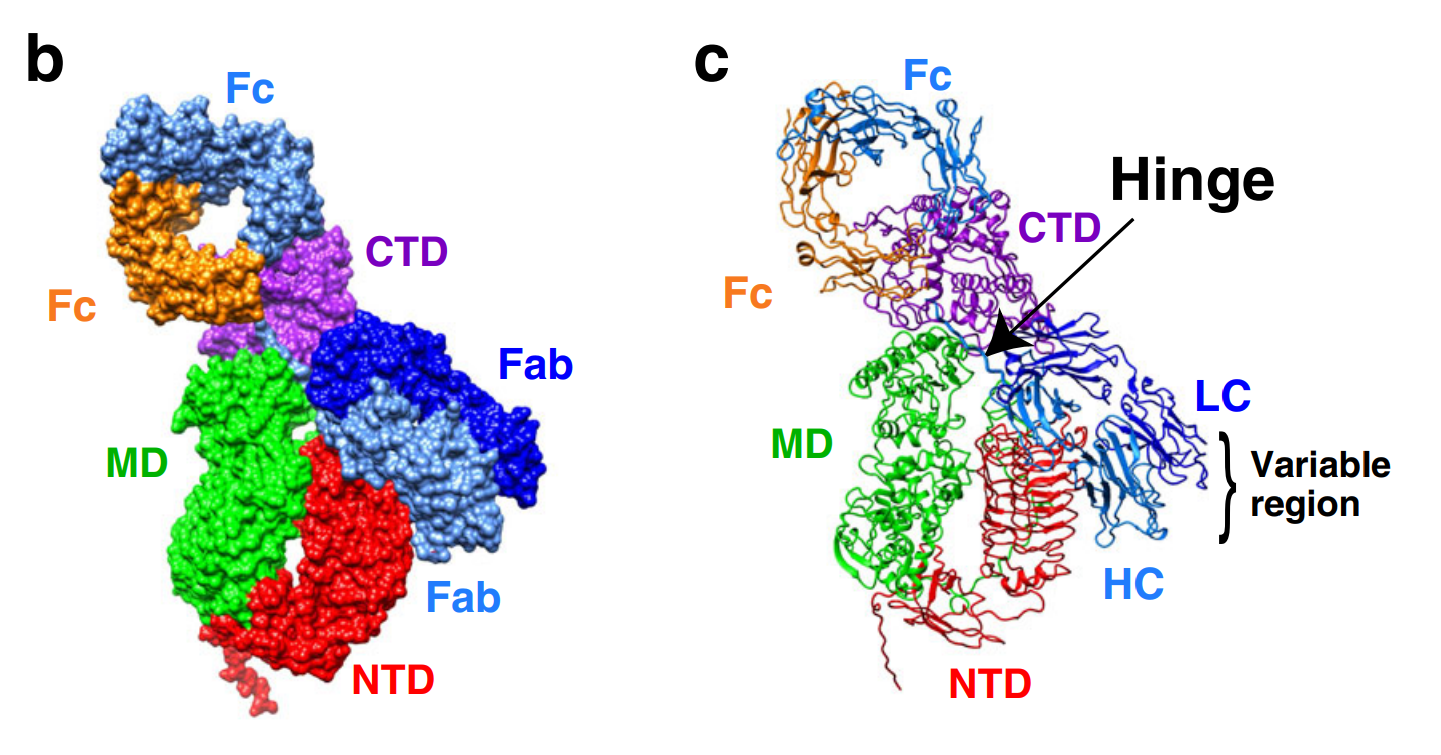
Immunoglobulin A1 protease (IgA1P)
Immunoglobulin A1 proteases (IgA1Ps) are a family of enzymatic virulence factors
secreted from a wide variety of pathogenic bacteria known to infect human mucosal surfaces. Once secreted, IgA1Ps cleave and inactivate the predominant mucosal antibody, immunoglobulin A1 (IgA1), to hinder the host’s adaptive immune response. As such, these enzymes are heavily implicated in these species’ pathogenicities. Efforts are therefore underway to understand how IgA1Ps select for and cleave IgA1 to aid in the development of drugs to treat bacterial lower
respiratory tract infections, among other clinical and biochemical applications.
Redzic JS, Rahkola J, Tran N, Holyoak T, Lee EJ, Martín-Galiano AJ, Meyer N, Zheng HJ, and Eisenmesser EZ. “A substrate-induced gating mechanism is conserved among Gram-positive IgA1 metalloproteases”. Commun. Biol. (2022). doi: 10.1038/s42003-022-04173-3.

Wang ZM, Rahkola J, Chi YC, Tran N, Holyoak T, Zheng HJ, Janoff E, and Eisenmesser EZ. “Mechanism and inhibition of Streptococcus pneumoniae IgA1 protease”. Nat. Commun. (2020). doi: 10.1038/s41467-020-19887-3.
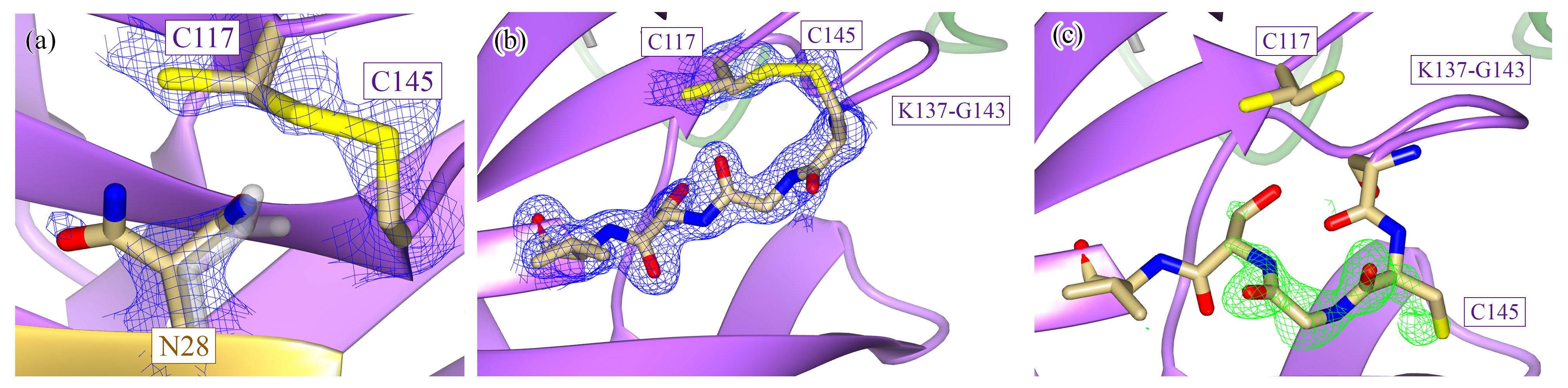
Phosphoenolpyruvate carboxykinase (PEPCK)
Our investigations of PEPCK utilize this enzyme family which encompasses GTP-, ATP-, and PPi-dependant enzymes as a model system in which to investigate and develop our understanding of the linkage between the conformational plasticity of protein structure and enzyme function and molecular recognition. Our studies on PEPCK have additional impacts upon human health as PEPCK is an important cataplerotic enzyme whose activity in humans and other mammals is essential to the maintenance of blood glucose levels. Consequently, flux through PEPCK contributes to the fasting hyperglycaemia observed in individuals afflicted with either Type I or Type II diabetes and has recently been implicated as a potential player in important biological processes such as cancer and aging.
McLeod MJ, Holyoak T. “Biochemical, structural, and kinetic characterization of PPi -dependent phosphoenolpyruvate carboxykinase from Propionibacterium freudenreichii”. Proteins.(2023);91(9):1261-1275. doi: 10.1002/prot.26513
Barwell SAE, Duman R, Wagner A, Holyoak T. “Directional regulation of cytosolic PEPCK catalysis is mediated by competitive binding of anions”. Biochem Biophys Res Commun. (2022) doi: 10.1016/j.bbrc.2022.11.025.
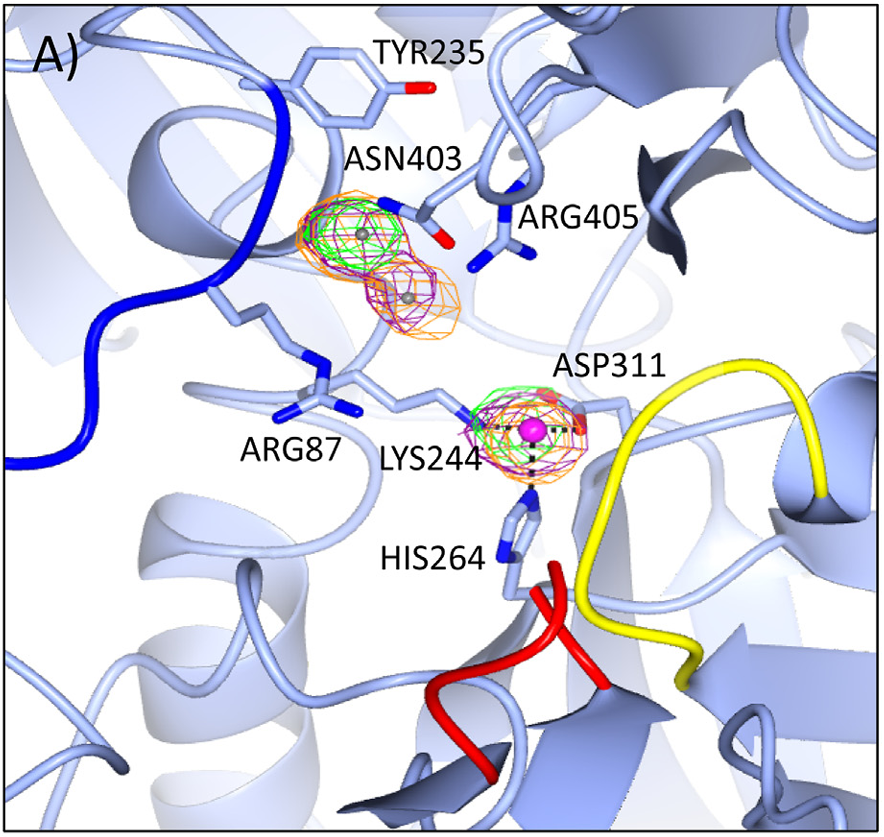
Cytidine triphosphate synthase (CTP synthase)
CTP synthase plays a pivotal role in nucleotide biosynthesis as the enzyme responsible for the de novo synthesis of CTP from UTP, a process that is essential for cellular function and proliferation. Through collaborative work with the Bearne Lab at Dalhousie University, we are interested in gaining a more complete understanding of the role of conformational dynamics and catalytic function as well as inhibition of this enzyme from various sources. Due to the essential nature of this enzyme in cellular function and proliferation, the knowledge gained through these studies holds immense potential in drug development, as CTP synthase dysregulation is implicated in various diseases, including cancer and neurodegenerative disorders as well as provides a potential avenue for the development of novel anti-microbial and anti-parasitic strategies.
McLeod MJ, Tran N, Gillis TD, McCluskey GD, Bearne SL, and Holyoak T. “A metal-dependent conformational change provides a structural basis for the inhibition of CTP synthase by gemcitabine-5’-triphosphate”. Protein Sci. (2023) doi: 10.1002/pro.4648.


Advancing crystallographic techniques
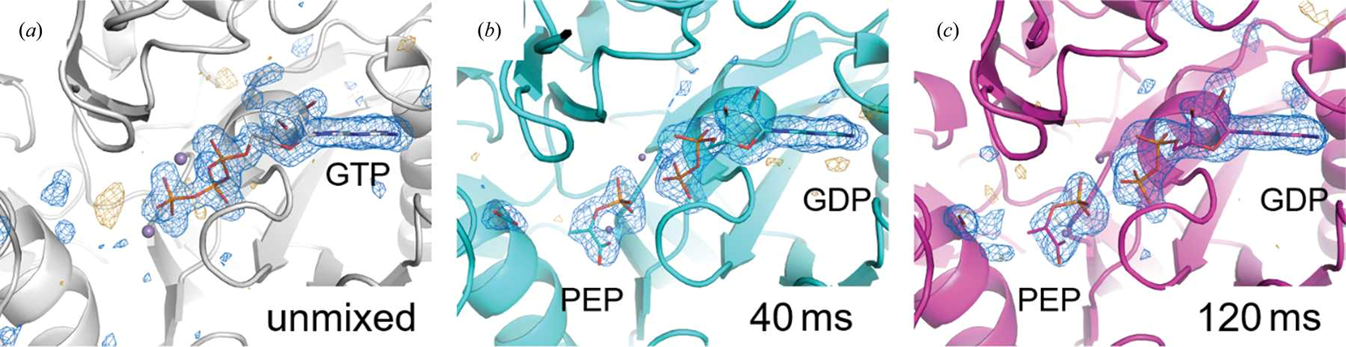
Structures traditionally determined by X-ray crystallographic approaches are obtained from crystals at ambient pressure and either cryogenic or room temperature. These structures represent time- or conformationally averaged structural ensembles. In collaboration with the Thorne Lab at Cornell University, we are determining high-resolution protein structures spanning temperature ranges from -180 to >40 °C as well as trapping intermediate states populated during enzymatic turnover at times less than 30 ms via freeze trapping approaches developed by the Thorne Lab (MMQX). Lastly, we are exploring the generation of novel insight into enzymatic mechanism and structure that can be gained by the determination of high-resolution enzyme structures at high pressures (>100 MPa) utilizing the facilities available at the Cornell High Energy Synchrotron Source (CHESS).
A structural perspective on the temperature-dependent activity of enzymes
Matthew J. McLeod, Sarah A. E. Barwell, Todd Holyoak, Robert Edward Thorne
bioRxiv 2024.08.23.609221; doi: https://doi.org/10.1101/2024.08.23.609221
Clinger JA, Moreau DW, McLeod MJ, Holyoak T, and Thorne RE. “Millisecond mix-and-quench crystallography (MMQX) enables time-resolved studies of PEPCK with remote data collection”. IUCrJ. (2021). doi:10.1107/S2052252521007053.

Fundamentals of enzyme kinetics
Tran N and Holyoak T. “Molecular Recognition: Lock-and-Key, Induced Fit, and Conformational Selection”. Encycl. Biophys. (2020). doi:10.1007/978-3-642-35943-9_468-1.

Student Posters
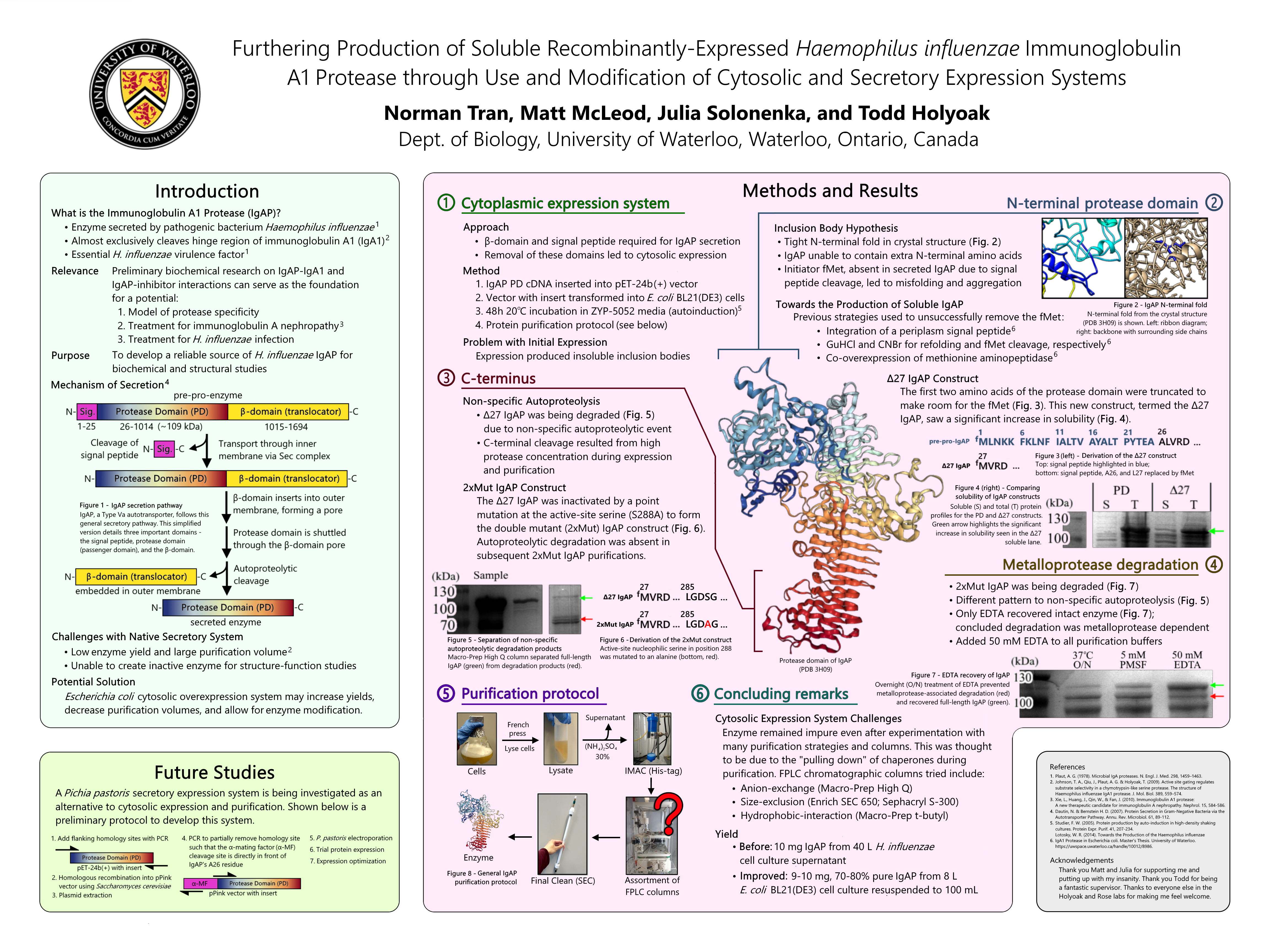
Haemophilus influenzae IgA1P
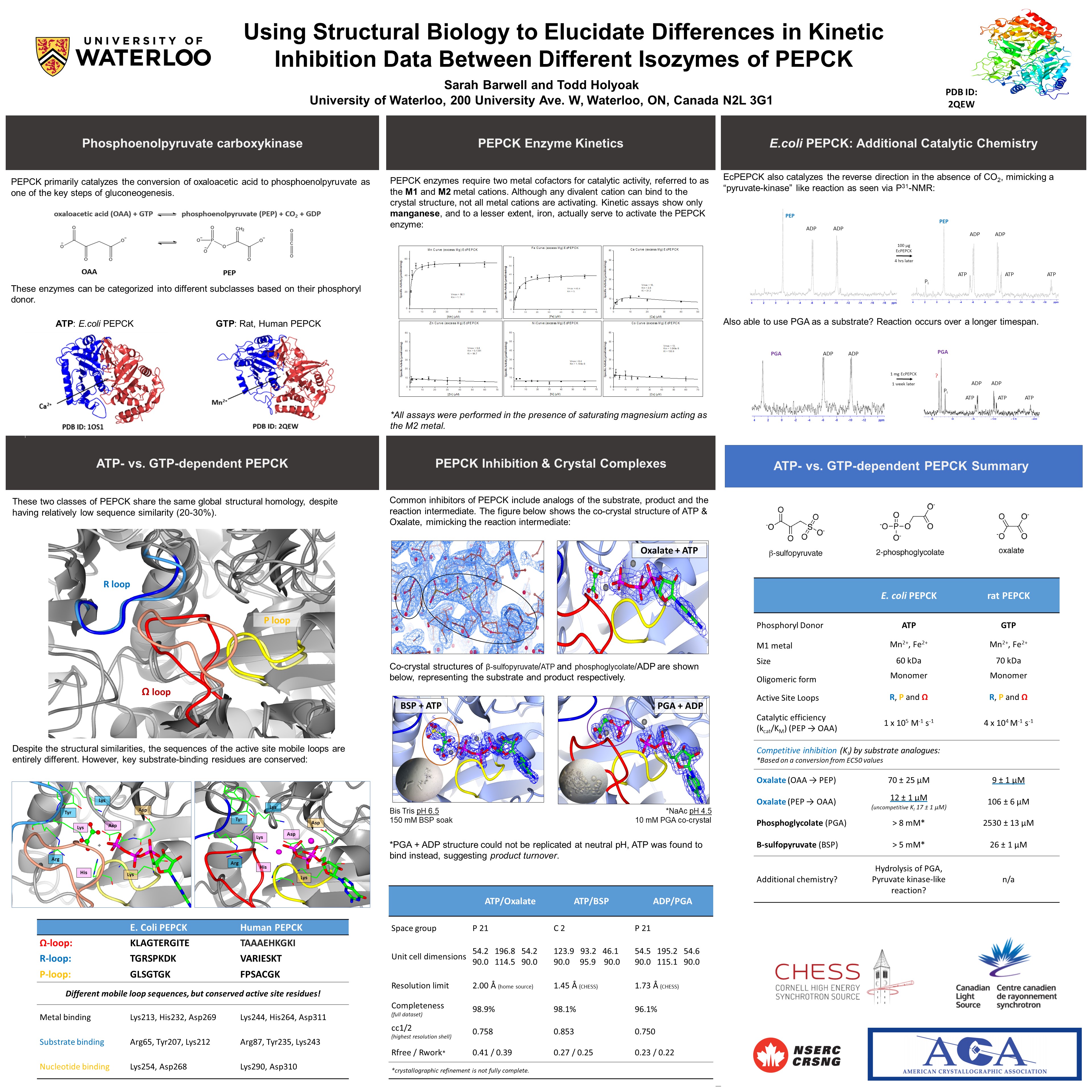
PEPCK
Sarah Barwell was awarded the Louis Delbaere Pauling Poster Prize for this poster.
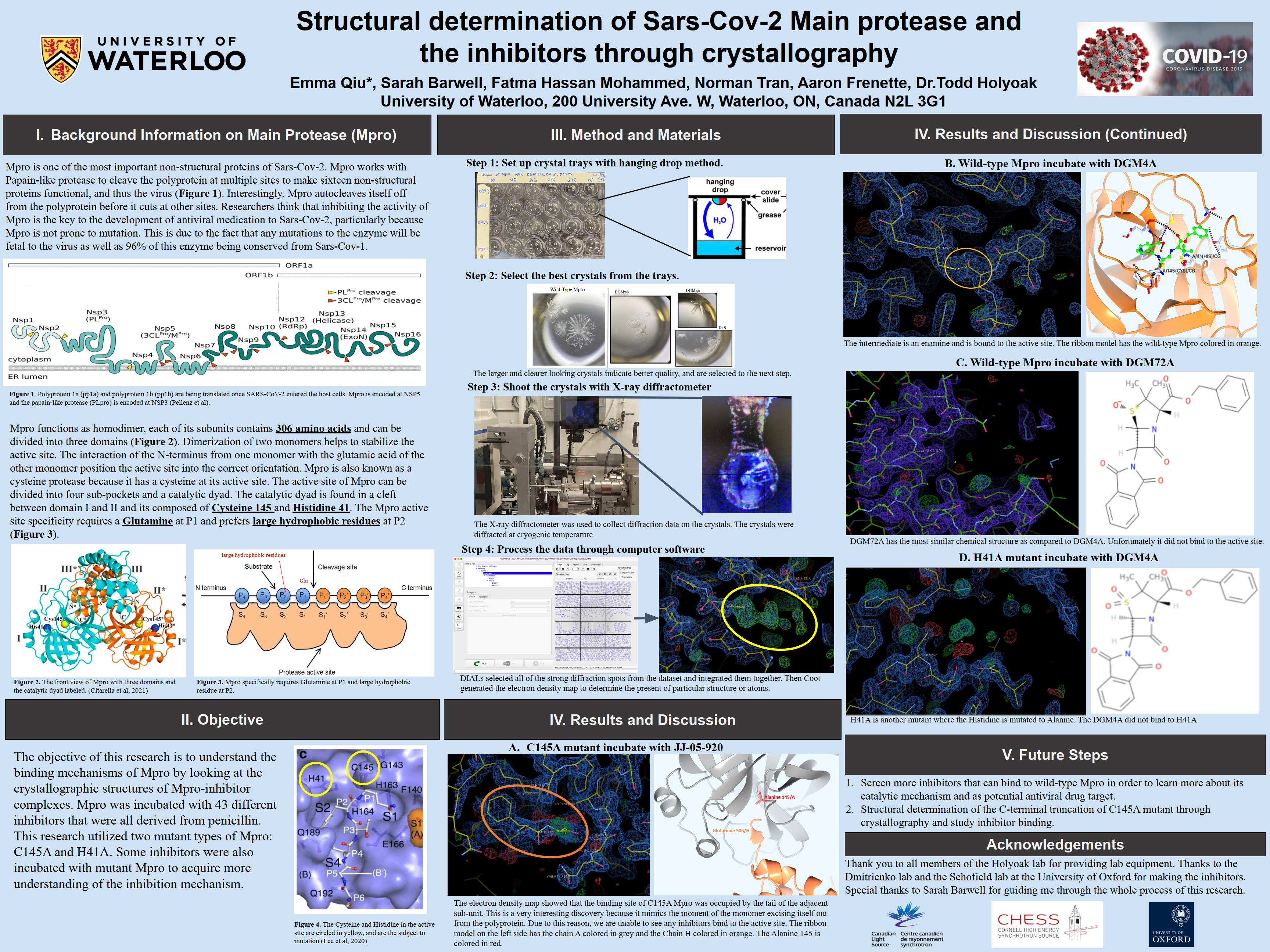
Mpro
Streptococcus pneumoniae IgA1P