EXPERIMENTAL EXPERTISE
Protein X-ray Crystallography
Macromolecular X-ray crystallography is the gold-standard method of experimentally determining a protein’s structure to atomic or near-atomic resolution. This technique requires a heavily specialized skillset spanning protein construct design, expression, purification, characterization, crystallization, and crystallographic data processing, each of which must be mastered to ensure the highest success for a project.
Specialized Instrumentation and Facilities
All steps of the structure-solution process require particular instruments and, especially near the end of the pipeline, specialized national facilities. These instruments include French pressure cells, sonicators, chromatography equipment, crystal-manipulation devices, etc.



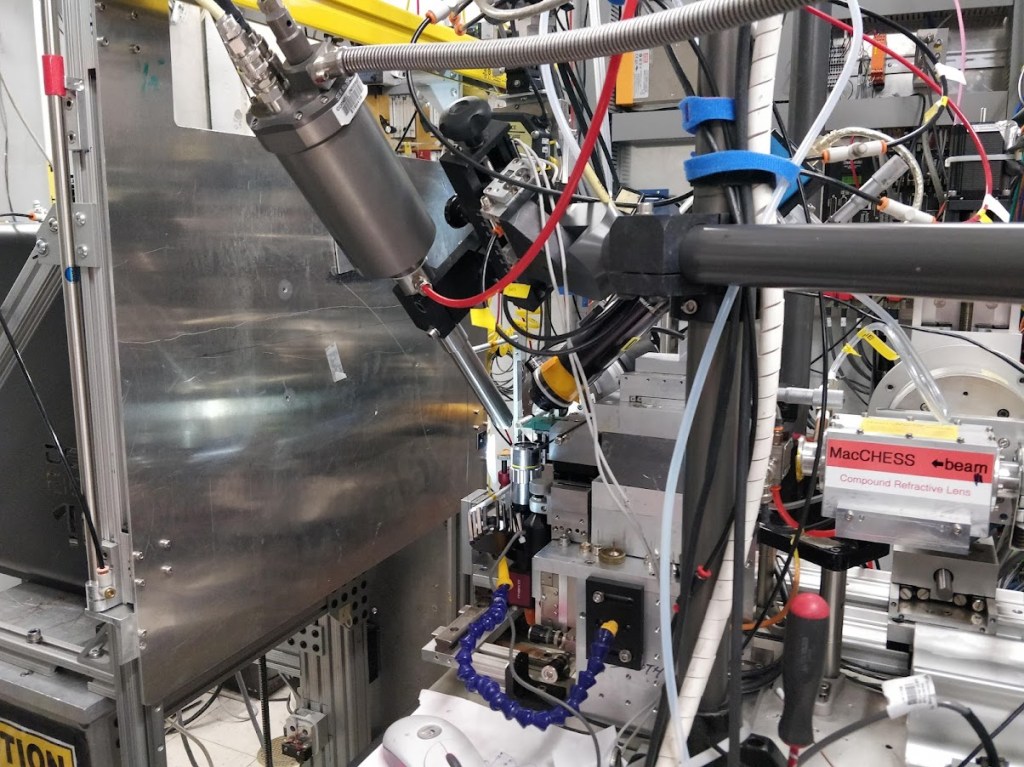
Data collection occurs at high-energy X-ray sources located at the University of Waterloo or at national facilities called synchrotrons found around the world. We primarily use the synchrotrons at the Canadian Light Source and Cornell High-Energy Synchrotron Source.

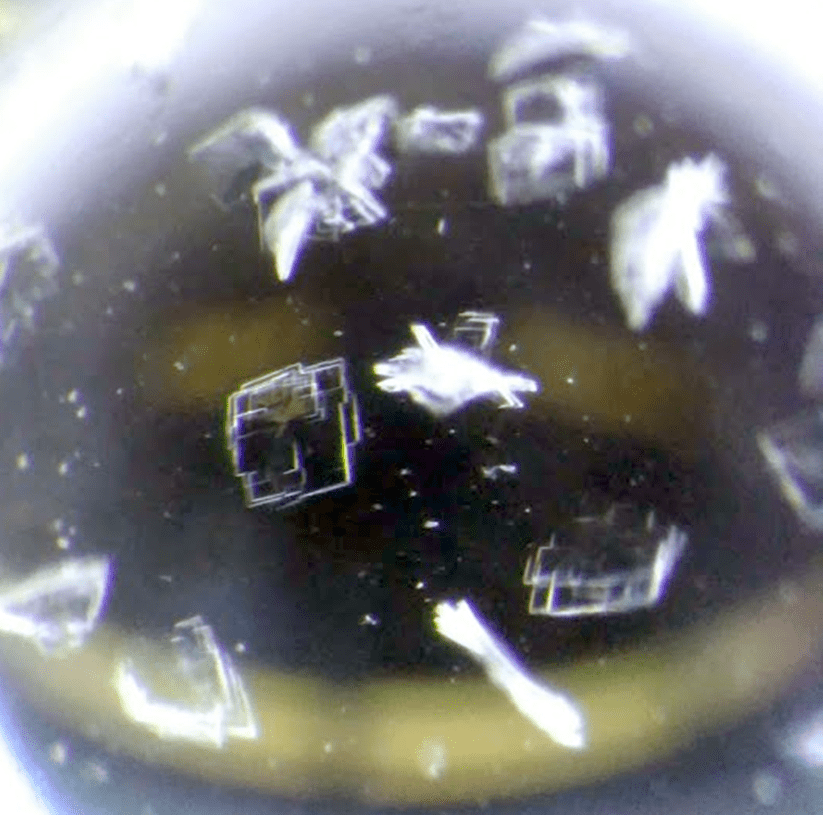
Protein Crystals
Proteins are crystallized under defined and reproducible conditions, leading to many different crystal morphologies. At times, protein crystallization can feel more like a skilled art than an exact science.





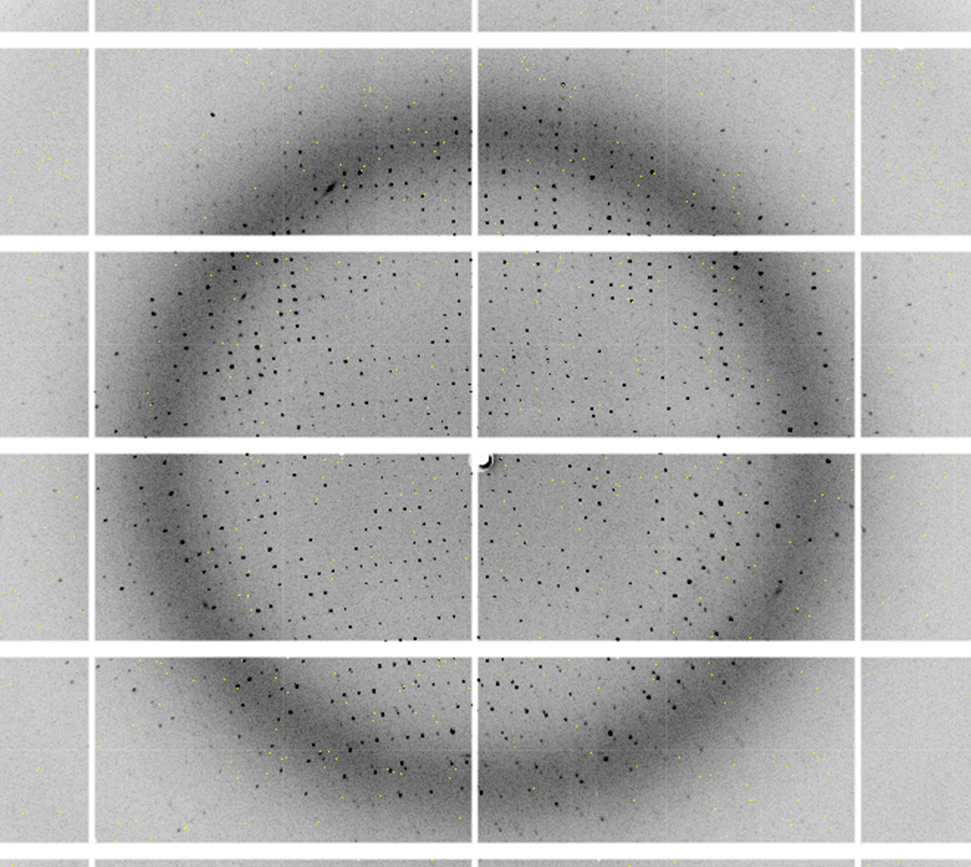
Diffraction Patterns
Protein crystals are shot with a high-energy beam of X-rays (homesource or synchrotron radiation) to generate a set of diffraction patterns which are subsequently processed to solve for the protein’s structure. The protein is crystallized to amplify the X-ray radiation to detectable levels.


Biophysical Protein Characterization
It is extremely important to ensure that the proteins that we produce are high-quality, reproducible, and behave well. We characterize and validate our proteins with a variety of biophysical methods and enzyme assays, if applicable. Some of these methods require synchrotron radiation.
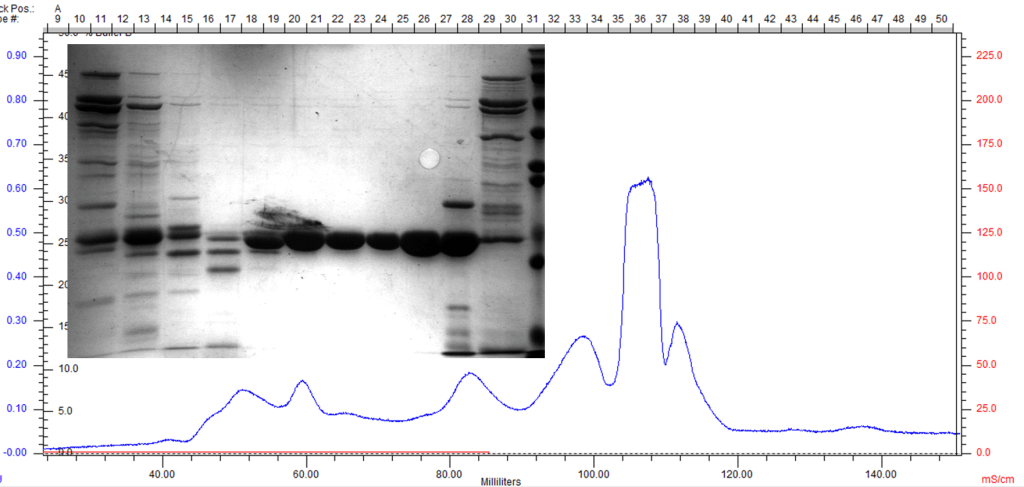
Size-Exclusion Chromatography
Size-exclusion chromatography (SEC) is the final polishing step of protein purification. The SEC column is able to separate proteins by hydrodynamic radii, removing soluble aggregates, misfolded protein, and contaminants all in one step. The following SEC trace exemplifies the separation of contaminants and the resulting purity.
Small-Angle X-ray Scattering
Small-angle X-ray scattering (SAXS) is a biophysical technique that utilizes the same physical principles of X-ray diffraction to determine the general size, shape, oligomerization state, foldedness, etc. of proteins in solution. This method requires synchrotron radiation and hence is done at the Cornell High-Energy Synchrotron Source.

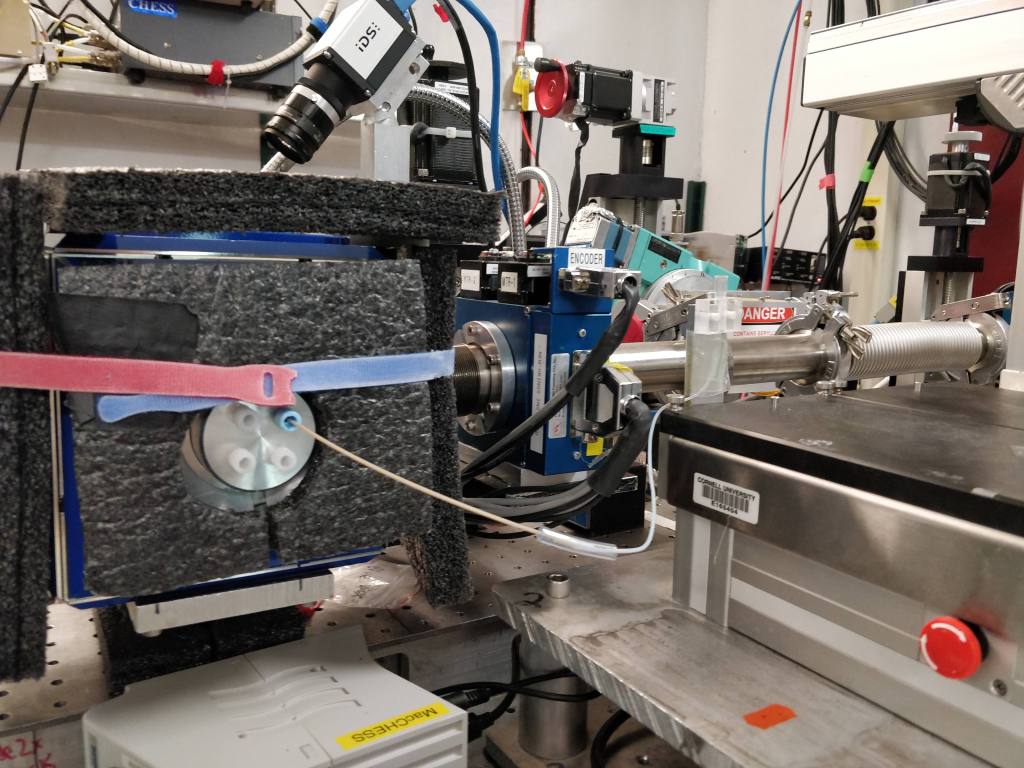

Biophysical Experiments
We have access to equipment at the university to perform differential scanning fluorimetry (DSF), differential scanning calorimetry (DSC), isothermal titration calorimetry (ITC), circular dichroism (CD), and dynamic light scattering (DLS) to obtain biophysical data about a protein’s oligomeric state and stability, to give a few examples.
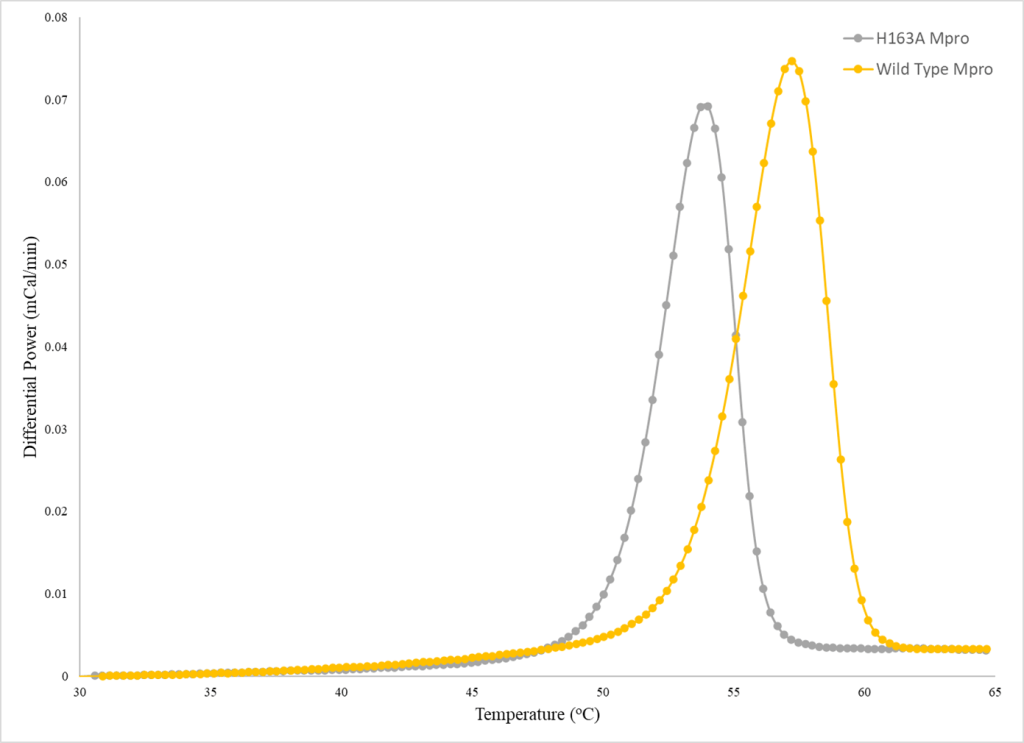
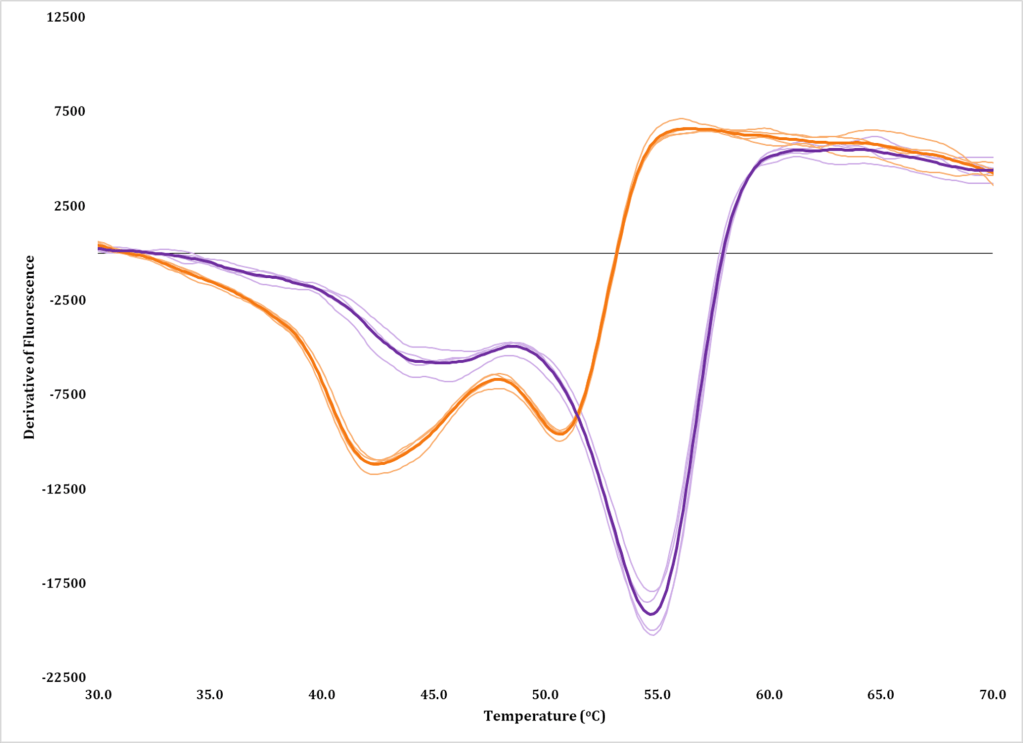
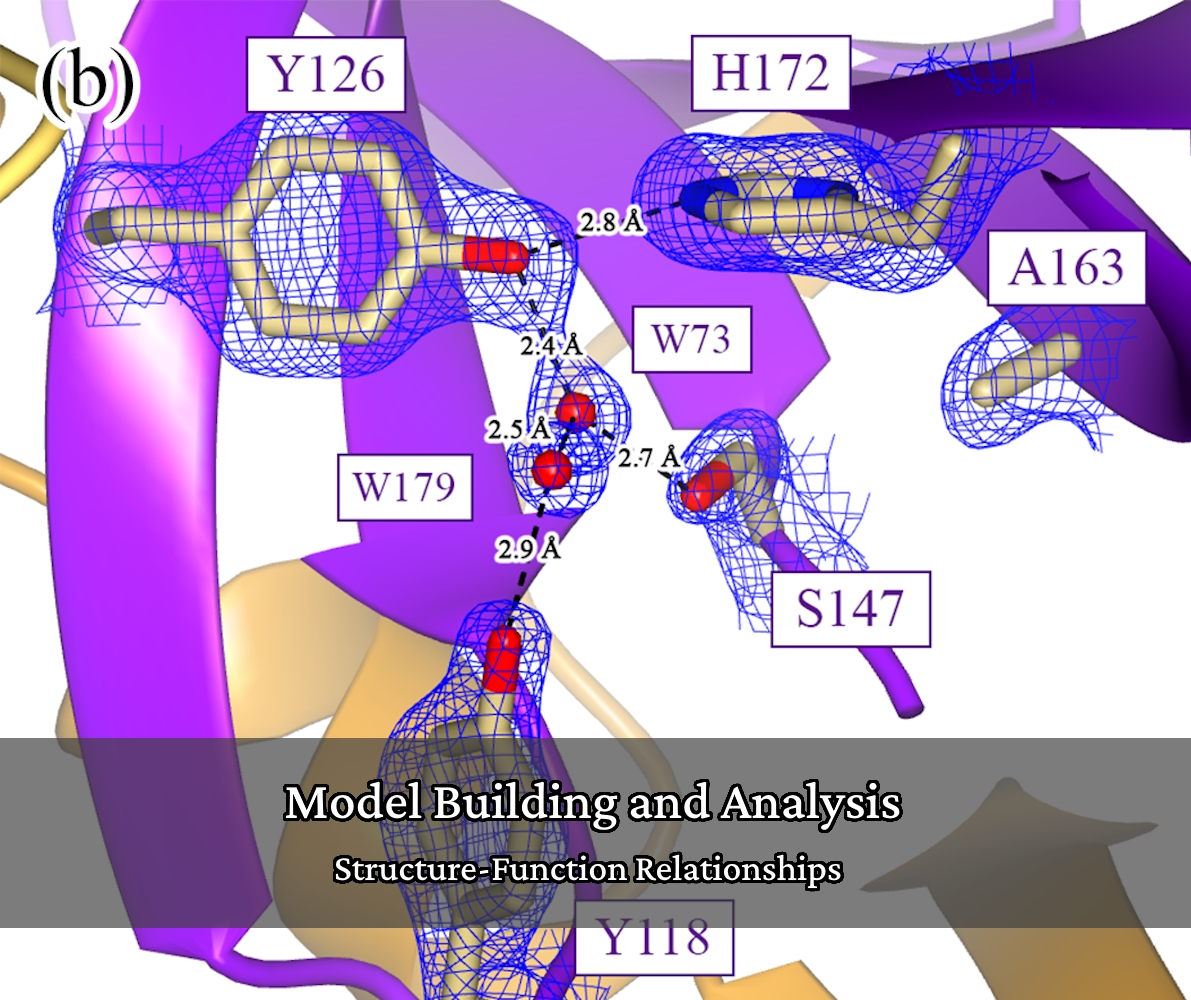
Structural Enzymology
Our research tries to understand how proteins, particularly enzymes, function and how that is influenced or dictated by the protein’s structure (structure-function relationships). We make mutations and/or truncations to protein constructs to test our hypotheses on structure-function relationships through enzyme assays that are developed and validated in-house.
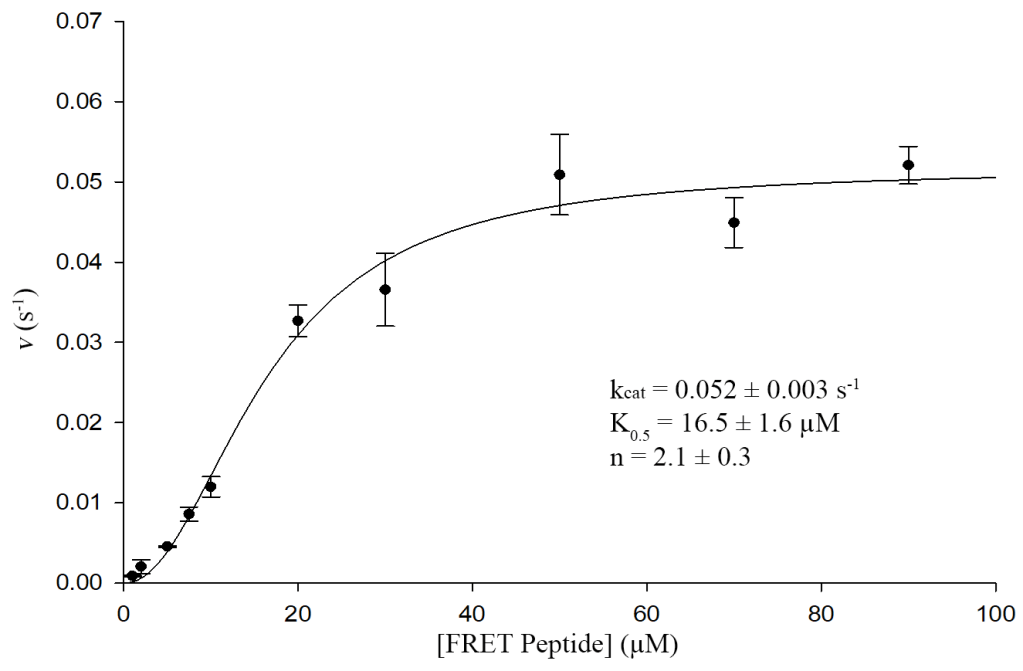
Enzyme Kinetics
We can measure enzyme activity through kinetic assays under steady-state initial-rate conditions and use these data to obtain kinetic constants (Michaelis-Menten kinetics). These constants can then be compared between protein variants to test our hypotheses. This experimental platform can also be varied to obtain other relevant information (e.g., IC50, inhibition constant, type of inhibition, etc.).